Research
How physical environments regulate biological matter remains an extensively explored, yet perpetually intriguing question. While biochemical and multi-omics approaches target specific molecular agents, biophysical approaches focus on the non-linear dynamics of living systems. However, recreating complex 3D physical milieus under experimentally-controlled conditions remains a technical challenge. Furthermore, such findings are either cell type/model organism-specific, and mostly implicate cell-intrinsic factors such as chemical signaling, metabolic modes, mechanotransduction, and inter-species interactions.
My PhD research pioneers a new perspective which establishes the physical microenvironment as an active regulator of biological matter across scales. By providing a foundational basis towards understanding the physico-chemical regulation of life forms, my work with Dr. Tapomoy Bhattacharjee elucidates generalizable physical principles describing how complex 3D environments regulate fundamental phenomena such as growth, motility, morphology, and cellular states.
Engineering platforms for 3D cell culture and 3D bioprinting

We have innovated custom-engineered 3D culture platforms that mimic the spatial architecture of habitats such as soil, mucus, and tissues – generating a class of universally-adaptable in vitro systems suitable for bioengineering and 3D bioprinting. We recently developed a high-throughput, cost-effective, and universally adaptable 3D culture platform, suitable for studying cellular growth, motility, and self-organisation across well-defined mechanical regimes. This also serves as a scaffold for 3D bioprinting tissue-like cell-ECM structures and establishing host-pathogen co-culture models. The key innovation here is a rapid and easily accessible synthesis strategy which uses commonplace reagents and bench top equipment, putting 3D cell culture within an arm’s (and minutes!) reach of any standard laboratory. Leveraging these, we have been investigating how active biological matter interacts with and responds to its surroundings - thereby bridging application-oriented 3D biomaterials manufacturing with fundamental biophysical questions.
-
Sreepadmanabh et al., - Jammed microgel growth medium prepared by flash-solidification of agarose for 3D cell culture and 3D bioprinting; Biomedical Materials (2023)
-
Sreepadmanabh*, Arun*, et al., - Design approaches for 3D cell culture and 3D bioprinting platforms; Biophysics Reviews (2024); selected as Featured Article
-
Das, Sreepadmanabh, et al., - Biophysical Design Space for Cellular Self-assembly and Dynamics; bioRxiv (2025)
-
Goswami, Mukherjee, Ahmed, Sreepadmanabh, et al., - Frequency Chirped Actuation of Chiral Magnetic Microbots for Viscometry Mapping in Heterogeneous Media: From Model Fluids to Living Cells; ACS Nano (2025)
Physical confinement as a selective pressure

For over three hundred years, microbiologists have looked at bacterial growth using experimental systems like liquid broths and flat plates. However, these do not capture the complexity of their natural habitats such as soil, infected tissues, and mucus. In our study, we bridged this gap by engineering mucus-like 3D systems to culture bacteria. Using this, we found that the structure of bacterial colonies in 3D space is dictated by the shape of a single cell - while rod-shaped bacteria form elongated and spread out colonies, spherical bacteria form compact and rounded colonies. Remarkably, this shape-dependent pattern allows rod-shaped bacteria to grow more successfully in highly confined 3D environments, helping them outcompete the spherical bacteria. Hence, our work provides the first-such experimental evidence that physical confinement plays a selective role in determining bacterial growth fitness. This completely alters the way we traditionally think about how microbial populations survive and adapt across diverse ecological settings such as soil, aquifers, mucus, and infected tissues. Importantly, our research provides a new framework for understanding how the mechanical properties of an environment can actively regulate its resident biological matter.
-
Sreepadmanabh et al., - Cell shape affects bacterial colony growth under physical confinement; Nature Communications (2024); selected as Editors' Highlight (top 50 papers in Microbiology and Infectious Diseases)
-
Bagade, Sreepadmanabh, et al., - Physico-chemical regulation of bacterial growth success under 3D confinement (under review)
Physical regulation of cellular division
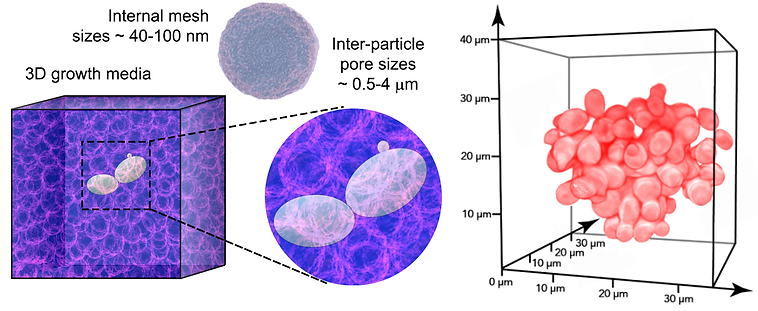
Molecular control over cell division is traditionally studied using liquid broths or 2D flat-plate cultures — neither of which recapitulate the complex visco-elasto-plastic properties of 3D natural habitats such as tissues, mucus, and soil. Consequently, how such regimes of physical confinement influence proliferative growth remains unknown. Here, by engineering mechanically tunable and transparent growth matrices, we directly visualize yeast budding across 3D viscoelastic regimes. We discover that elevated physical confinement drastically prolongs budding intervals without causing physiological defects. Remarkably, reduced proliferative rates are not associated with transcriptional signatures of mechanosensation or cell cycle dysregulation. Rather, 3D confinement physically constrains the volumetric growth of incipient buds — manifesting as delayed cell cycle progression. Hence, our findings establish a fundamentally unique form of physical regulation over eukaryotic cell division.
-
Sreepadmanabh*, Gautam*, et al., - 3D confinement physically regulates cell cycle progression in budding yeast; bioRxiv (2025)
Confinement-regulated transitions in motility

The natural habitats of nematodes are three-dimensional granular niches with complex material properties that impose mechanical constraints on their motion. However, laboratory studies typically employ liquid cultures or 2D agar pads. Here, we show that 3D physical confinement dramatically alters the nature of undulatory motion by inducing gait changes that maximize propulsive efficiency. By engineering mechanically tunable, transparent 3D granular matrices, we find that the propulsive speed of nematodes shows a non-monotonic dependence on the yield stress of their microenvironment. Direct visualization, biophysical measurements and theoretical predictions collectively reveal that under high confinement, nematodes optimize for efficient motion by matching forward propulsive speed to the wave speed along their body. Further, in a non-dimensionalized phase space defined by propulsive efficiency and time scale of motility, increasing confinement leads to sharp motility transitions from poorly efficient thrashing to highly efficient crawling. Our work establishes a biophysical paradigm wherein distinct modes of undulatory motion emerge as a consequence of 3D physical confinement.
-
Sreepadmanabh*, Dey*, et al., - Physical confinement regulates transition in nematode motility; PRX Life (2025)
Chemical cues reprogram cellular and multicellular phenotypes

The self-organization of cellular collectives is crucial in development and cancer. Multicellular aggregation in cancer is associated with a higher efficiency of metastasis. However, we do not fully understand how mechanochemical microenvironmental cues affect the organization and stability of such ensembles. Here, using a model system of ovarian cancer spheroids, which temporally transit from solid, dysmorphic moruloids to structurally plastic, lumen-containing blastuloids, we show that the periodic volume fluctuations observed in blastuloids are driven by lumenal fluid influx and cell-cell junctional states. Furthermore, blastuloid cell states are reprogrammed, which enables them to rapidly recover from even complete structural disintegration and self-organize into fully lumenized ensembles. Using targeted chemical perturbations, we identify two distinct cues regulating separate transition traits: calcium levels establish cell states cognate to, and pH regulates the fluctuation dynamics of, blastuloid phenotypes. Our work holds significant implications towards understanding mechanisms governing structural resilience and plasticity in complex cellular assemblies.
-
Sreepadmanabh et al., - Distinct chemical cues reprogram cellular and multicellular phenotypes in ovarian cancer spheroids; Small (2025)
Dissecting plant-fungal pathogenesis using synthetic 3D soil

Understanding plant-fungal interactions is crucial for interrogating host-symbiont and host-pathogen dynamics, with significant fundamental and translational implications. Although plants and fungi typically inhabit soil – an opaque, disordered, and granular 3D environment – our current understanding of molecular dialogues largely stems from experimental systems employing 2D flat plates or hydroponic cultures. Here, we introduce a transparent 3D soil-like granular matrix, which enables direct cellular level visualization and long-term interrogation of plant-fungal interactions. We track fungal colonization of plant roots with unprecedented 3D resolution as well as integrate bulk transcriptomic analyses to discover novel gene regulatory programs activated solely under conditions of 3D culture - representing a tranche of unique molecular signatures likely associated with biomimetic soil-like environments. Together, our findings establish a powerful and versatile 3D platform for investigating plant-fungal interactions. These insights hold immense potential for advancing our understanding of plant immune responses and fungal pathogenicity pathways, as well as for the development of drought and disease-resistant cultivars with major agricultural benefits.
-
Thomas*, Sreepadmanabh*, et al., - Dissecting the root-fungal interface in 3D reveals spatially distinct signalling landscapes; bioRxiv (2025)